Talk about a “Golden Delicious apple,” and everyone understands that the name refers to the fruit’s color, not its composition.
Talk about “ruby red grapefruit,” and the situation’s the same.
Talk about “silver fillings,” though, and things get a little more complicated.
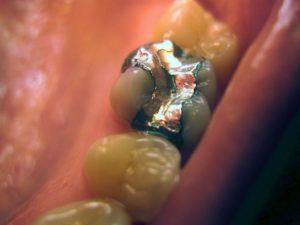 After all, a metal like gold or a precious stone like a ruby would never be confused with a piece of fruit. They’re entirely different things. But call it a “lemon apple” or a “cherry grapefruit,” and you probably start thinking about hybrids like tangelos and pluots.
After all, a metal like gold or a precious stone like a ruby would never be confused with a piece of fruit. They’re entirely different things. But call it a “lemon apple” or a “cherry grapefruit,” and you probably start thinking about hybrids like tangelos and pluots.
The case is similar when you call one metal by the name of another. It’s misleading.
And this is the focus of a report released just last week by Consumers for Dental Choice. Based on a new national poll by Zogby Analytics, the report provides critical evidence showing what happens when you don’t call amalgam fillings what they really are: mercury amalgam fillings.
Dental amalgam is about 50% mercury, a potent neurotoxin.
Most Americans – 57% – don’t know this, Zogby found. When asked to identify the main material in amalgam, most (23%) said silver.
Even more troubling is the fact of how few patients are ever told by their dentists that amalgam fillings are mostly mercury: just 11%. A nearly 2/3 majority feel they are not given enough information about alternative materials to make informed decisions about their care.
And the kicker? Women are even less likely than men to say they’re sufficiently informed. Consider that plenty of women – such as the one featured in this recent piece from WDHD News in Boston – do what they can to reduce their exposure to mercury when pregnant but miss a major source if they don’t know what their dental work is made of.
Informed consent is impossible in such circumstances.
“The root pin of the problem,” says Charlie Brown of Consumers for Dental Choice, “is the US Food and Drug Administration, who allows the dental industry to conceal the fact of the mercury from dental patients and who is violating its own rules by allowing this deceptive marketing, approving the cover-up.”
That’s some strong language, but the truth of the matter is this: Section 502 of the Federal Food, Drug and Cosmetic Act – the act that founded the FDA – requires that the agency keep companies from putting “incorrect, inadequate or incomplete” labels on their products. But as Charlie noted upon releasing the new report, the term “silver” is incorrect, and “amalgam” is incomplete. Consequently, both are inadequate.
Of course, this has been an issue for years. Why bring it special attention now? The Minamata Convention on Mercury, which Charlie refers to as “the game-changer.” The US was the first country to ratify this global treaty – a treaty which puts the lie to the FDA’s insistence, against science, that a potent neurotoxin is perfectly harmless when implanted into living tissue mere inches away from the brain.
This graphic from the Consumers report illustrates the conflict very well:
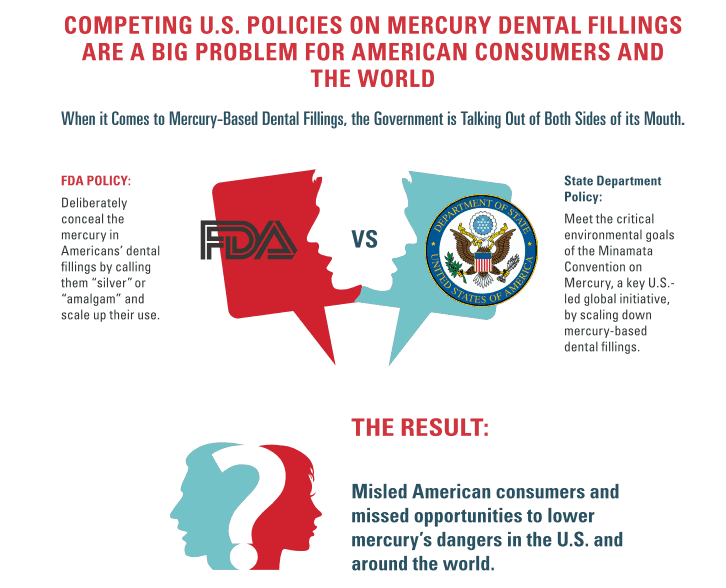
Thus, the new call to action, urging the FDA to finally start holding the makers of dental amalgam to the standards it holds others to, to stop misleading and allowing consumers to be misled.
To this end, Consumers for Dental Choice has launched a new petition to Secretary of State John Kerry to help bring the FDA in line with our nation’s official stance against mercury. We encourage you to take a moment to sign and share the petition as widely as you can.