Our thanks to the office of biological dentist Dr. Gary Verigin for letting us re-post this recent piece from their blog Know Thy Health:
 As long as we’re in this mode of updating past posts, we thought to take a look at the continuing research on how tooth bleaching may affect mercury amalgam fillings.
As long as we’re in this mode of updating past posts, we thought to take a look at the continuing research on how tooth bleaching may affect mercury amalgam fillings.
For there’s good reason for extra concern.
The most recent study, like many that came before, involved exposing amalgam samples to either peroxide or a neutral fluid and measuring mercury release. In this case, two types of amalgam disks were used for testing, one with a much higher silver content than the other. Both were exposed to the peroxide or control fluid for 48 hours. The amount of mercury dissolved in each test tube was then measured.
The results? Peroxide stimulated more mercury release. And the less silver, the more mercury released.
This is in line with the research we told you about before, as well as later research, such as the 2013 General Dentistry study, which showed that “exposing amalgam alloys to bleaching agents released greater amounts of [mercury] compared to exposing samples to deionized water.”
That said, there are some studies that have found no significant change in mercury release, but as a 2015 literature review noted, there may be good explanations for the discrepancies. For instance,
This controversy might be related to the variation in peroxide concentration and time period of application. An alternative hypothesis is that there is a positive correlation between the mercury release and peroxide concentration and the increased release of mercury is attribute to the age of the dental amalgam, the surface roughness of the amalgam surface and the acidity of the bleaching agent.
Of course, it helps when the research is sound. It isn’t always.
For instance, a 2014 study in the Journal of Esthetic and Restorative Dentistry concluded that
Bleaching treatments either office or home did not affect the amount of mercury released from amalgam fillings in blood, urine, and saliva and the antioxidant-enzyme activities in blood.
There are a number of problems with this study, however.
For one, it was a very small study with no control group.
It’s also worth bearing in mind that this is a one-time exposure, whereas folks who bleach tend to do so repeatedly in order to retain the degree of whiteness that made them turn to bleaching in the first place. We’re aware of no studies that show the potential effects of mercury release under repeated exposures over longer periods of time.
But we might also question whether mercury levels in blood, urine, or saliva are actually the best measure in this case.
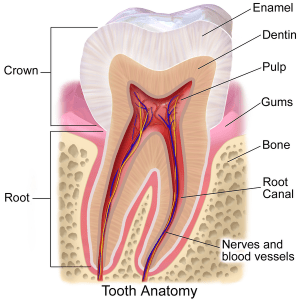 Researching caries (tooth decay) susceptibility and resistance back in the 1960s, Dr. Ralph Steinman of Loma Linda University found that peroxide appeared to reverse the flow of fluid through the miles of microscopic tubules that make up the middle layer of your teeth, the dentin. Normally, this fluid flows outward, repelling microbes and other threats to the health of the tooth. When the flow is reversed, these threats are pulled into the tooth. (You can read more about Steinman’s research here.)
Researching caries (tooth decay) susceptibility and resistance back in the 1960s, Dr. Ralph Steinman of Loma Linda University found that peroxide appeared to reverse the flow of fluid through the miles of microscopic tubules that make up the middle layer of your teeth, the dentin. Normally, this fluid flows outward, repelling microbes and other threats to the health of the tooth. When the flow is reversed, these threats are pulled into the tooth. (You can read more about Steinman’s research here.)
So if Steinman’s work is correct, why would we be amazed that mercury isn’t released into saliva, blood, or urine? The mercury would be in the dentinal tubules.
And the dentinal odontoblasts – specialized cells that form new dentin and which extend into the pulp – would be bathed in mercury.
Suffice it to say, this is not a good situation to put your teeth in, considering the neurotoxic effects of mercury and the very short distance between your teeth and brain.
It is good reason to steer clear of peroxide if you still have amalgam fillings.
Tooth anatomy image by BruceBlaus, via Wikimedia Commons